By the preparation of colored paint, a good dispersion quality is one of the most difficult factors. The dispersion process consists of converting dry pigments into pigment dispersion, which must be fine and sufficiently stable to achieve the final coloristic properties and stability. This is a complex process there resin, type of pigment, solvents and the use of dispersing agents are playing here an important role.
1. Dispersion process
The dispersion process of a pigment in liquid coatings can be divided into the three processes:
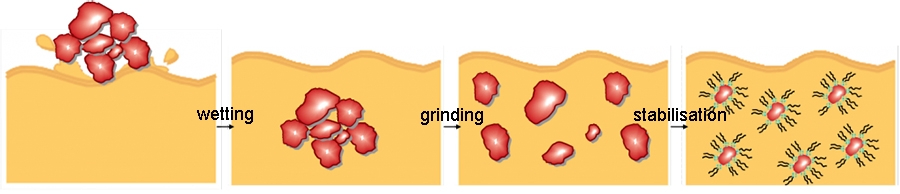
Wetting
The air and moisture covering the pigment is replaced by the resin solution. The solid/gas interface (pigment/air) is transformed into a solid/liquid interface (pigment/resin solution).
Grinding stage
By high shear forces the pigment agglomerates are broken up into smaller units, preferable primary particles.
Stabilization
The pigment dispersion is stabilized by dispersing agents in order to prevent the formation of uncontrolled flocculates. The resultant suspension is stabilized due to the adsorption of binder species or molecules at the pigment surface.
Pigment wetting: The air and moisture covering the pigment is replaced by the resin solution. The solid/gas interface (pigment/air) is transformed into a solid/liquid interface (pigment/resin solution).
Grinding stage: By high shear forces the pigment agglomerates are broken up into smaller units, preferable primary particles.
Stabilization: The pigment dispersion is stabilized by dispersing agents in order to prevent the formation of uncontrolled flocculates. The resultant suspension is stabilized due to the adsorption of binder species or molecules at the pigment surface.
Dispersing additives, which adsorb on the pigment surface, facilitate liquid/solid interfacial interactions and help to replace the air/solid interface by a liquid medium/solid interface.
The grinding process can be regarded as a de-flocculation process. In the absence of stabilizing agents, effects such as reduced color strength, decreased gloss, and altered rheology may occur.
1.1 Stabilization of pigment dispersions
The pigment dispersion what is achieved in the last step will be used later in the let down system where it should stay stable during storage and later during the application and film formation.
There are two principal mechanisms for the stabilization of pigmented dispersions described:
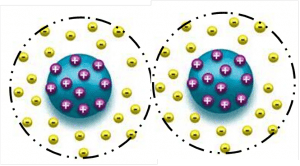
1.1.1 Electrostatic stabilization is only working in a water based application. When two particles having the same charges approaching each other will result in a repelling effect. The resulting Coulomb-repulsion of the charged particles allows the system to remain stable.
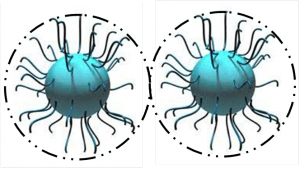
1.1.2 Steric stabilization suited for water and solvent based systems is when pigments are sterically stabilized (the surface of the solid particles are completely covered by polymers) making particle-to-particle contact impossible. Strong interactions between polymers and solvents (organic solvent or water) prevent the polymers from coming too closely into contact with one another (flocculation).
Steric stabilization relies on the adsorption of a layer of resin or polymer chains on the surface of the pigment.
One fundamental requirement of steric stabilization is that the chains are fully solvated by the medium. This is important because it means the chains will be free to extend into the medium. In systems where the chains are not so well solvated they will prefer to lie next to each other on the surface of the pigment, providing a very much smaller barrier to inter-particulate attraction what will result in much easier flocculation
2. Dispersants Families
The choice of the dispersing agents for the pigment stabilization is a key issue in the coating and ink industry. Formulators have to find the most suitable products for their formulation taking into account the final application of their coating, the coating system (water based, solvent based, etc.) and the other additives.
The role of the dispersing agents is to enhance the dispersion process and ensure a fine particle size in order to stabilize pigments in the resin solution. As explained earlier, an efficient dispersant has to perform the three main functions: pigment wetting, dispersing, and stabilizing. Dispersing agents generally differ for aqueous and solvent-based coatings.
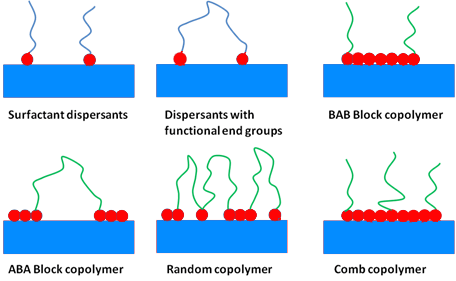
In term of chemical structure one can divide dispersing agents into the two following classes:
- Surfactants, also called low molecular weight dispersing agents
- Polymeric dispersants, also called high molecular weight dispersants
The main differences of those two types of dispersants being the molecular weight, the stabilization mechanism and the resulting let down stability.
In addition polymeric dispersing agents have multiple anchor groups where surfactant like dispersing agents more related to a polar head with a side chain for the compatibility.
2.1 Polymeric Dispersants
Polymeric dispersants stabilize paints, coatings and ink systems via a steric stabilization mechanism.
They must have specific anchor groups capable of being strongly adsorbed into the particle surface and must contain polymeric chains that give steric stabilization in the required solvent or resin solution system.
Polymeric dispersants differentiate themselves from the other types of dispersing agents through considerably higher molecular weights. Because of its structural features, a polymeric dispersant is bound to numerous sites at the same time, forming durable adsorption layers upon many pigment particles. Optimal steric stabilization is achieved when the polymer chains are well solvated and properly stretched, therefore they must be highly compatible with the surrounding resin solution. If this compatibility is obstructed, the polymer chains collapse causing the steric hindrance and the resulting stabilization to be lost.
In order for additives to be effective, the adsorption into the pigment surface must be durable and permanent. The surface properties of the pigment particles are therefore crucial to the additive’s effectiveness:
With pigments possessing high surface polarities, such as inorganic pigments that are ionically constructed, the adsorption of any dispersing additive is relatively easy.
However, for pigments with nonpolar surfaces, such as organic pigments whose crystals are composed of nonpolar individual molecules, a proper adsorption is rather difficult to obtain with conventional additives. The wide range of anchor groups that polymeric dispersants provide make them very efficient to anchor on pigments with nonpolar surfaces.
In the traditional method of stabilizing pigments in water, the stabilizing charges used are often disturbed by impurities, such as other ions, or the presence of other pigments with different zeta-potentials. This leads to a destabilizing effect, caused by the reduction of the repulsive forces. Steric stabilization can avoid this issue, making polymeric dispersants very useful for dispersing all types of pigments, even the organic ones, that are very difficult to be deflocculated by traditional wetting and dispersing additives.
The nature of the polymeric chain is critical to the performance of polymeric dispersants. If the chains are not sufficiently solvated, then they will collapse on to the pigment surface allowing the particles to aggregate or flocculate. The need for compatibility with the medium extends throughout the final drying stages of any applied coating. If it ceases to be compatible, flocculation may occur leading to a decrease of surface properties such as losses in gloss and tinctorial strength, etc.

The molecular weight of the polymeric dispersant must be sufficient to provide polymer chains of optimum length to overcome Van de Waals forces of attraction between pigment particles
Finally, for good surface coating properties and performances, the polymer must be fully compatible with the coating resin after the solvent has evaporated off and the resin has been cross-linked.
2.2 Low molecular weight dispersants (surfactants)
Surfactant dispersants are conventional low molecular weight dispersing agents. Surfactant molecules are able to modify the properties and, in particular, they lower the interfacial tension between the pigment and the resin solution.
This surface activity arises because the surfactants’ structure consists of two groups of contrasting solubility or polarity. In aqueous systems, the polar group is known as a hydrophilic group and the non-polar group as hydrophobic or lipophilic. In non-aqueous systems, the polar group is known as the oleophobic group and the non-polar group as oleophilic. Surfactants are classified according to their chemical structure and, more specifically, their polar group: anionic, cationic, electroneutral and non-ionic.
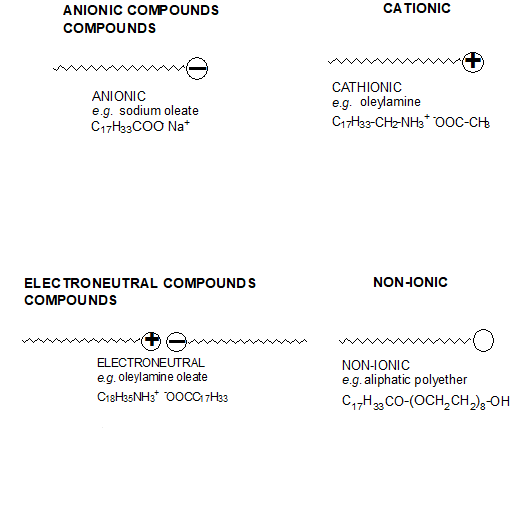
As with the polymeric dispersing agents, their effectiveness is determined by:
- The absorption of the polar group onto the pigment surface. The anchoring groups can be amino, carboxylic, sulfonic, phosphoric acids or their salts.
- The behaviour of the nonpolar chain in the medium surrounding the particle. This part of the molecule (aliphatic or aliphatic-aromatic segments) must be highly compatible with the binder system.
The stabilisation mechanism of surfactant-like dispersing agents is electostatic: the polar groups forming an electrical double layer around the pigments particles. Due to the Brownian movement the pigment particles frequently encounter each other in the liquid medium thus having a strong tendency to re-flocculate on the let down stage.
Because of their chemical structure (eg: low molecular weigth) and the electrostatic method of stabilization, surfactants may cause the following defects:
- Water sensitivity: Surfactants generally have a tendency to provide water sensitivity to the final coating, thus making them inappropriate for use in outdoor applications.
- Foam formation: Many surfactants generate foams which lead to surface defects (eg. fish eyes, craters) on the final coating. If foaming occurs at the milling stage it can also cause a loss of production capacity.
- Interference with intercoat adhesion.
Over the past years specific surfactants have been developed to minimize these defects, and
some provide other advantages to the final paints such as defoaming/dearation or difficult substrate wetting.
The most widely used surfactants for pigment dispersion in coating formulations are:
- Fatty acid derivatives
- Phosphate esters
- Sodium polyacrylates / polyacrylic acid
- Acetylene diols
- Soya lecithin
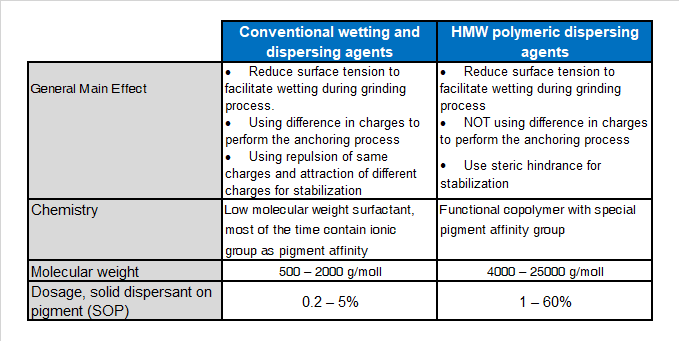
Main difference between LMW dispersant and HMW dispersants are:
3 Required amount of dispersant
Dispersing agents are not just additives to conventional millbases. The choice of the most suitable dispersing agents is sometimes difficult and their usage require sometimes specific guidelines.
The choice of dispersant is also related to the surface nature of the pigment. The polarity of the surface of the pigment differs from organic (non-polar) to inorganic (more polar), and this means that the nature of the dispersant anchor group is critical for optimum absorbtion. The choice of anionic anchor group should allow for better performance with inorganic pigments and a cationic anchor group should be more appropriate for organic pigments.
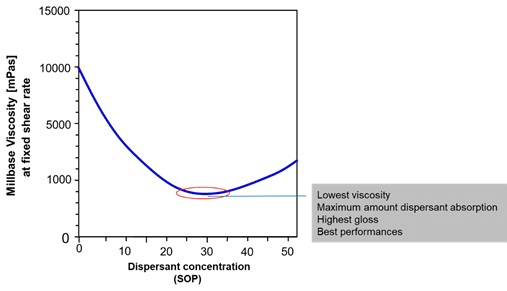
The surface area of the pigment also affects the level of dispersant used, and in general, if too little is used then the full benefits will not be realised. If too much is used, it can be shown that the thickness of the protective barrier is actually reduced as a result of overcrowding on the pigment surface. Therefore the use of an excess level of dispersant actually leads to final coating properties which are inferior to those obtained with an optimised dosage.
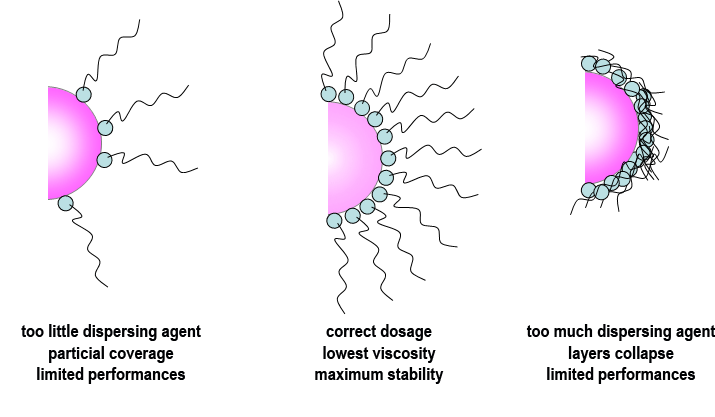
As a general rule, 2-2.5mg of polymeric dispersant, per square meter of pigment surface area will be close to the optimum amount required.
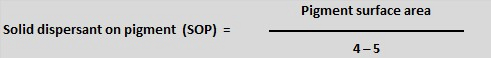
A ladder series of polymeric dosage levels should be evaluated based around this 2-2.5mg/m2 level. Measurement of dispersion viscosity will show a minimum at the optimum dosage; although it is also possible to measure gloss or colour strength of the coating which will show a maximum at the same optimum dosage.
![[:en]UNIQCHEM[:zh]website2[:]](https://www.uniqchem.com/wp-content/uploads/2019/04/cropped-Uniqchem-logo-final-no-background-2-2-e1675623758898.png)